Neonatal TSH
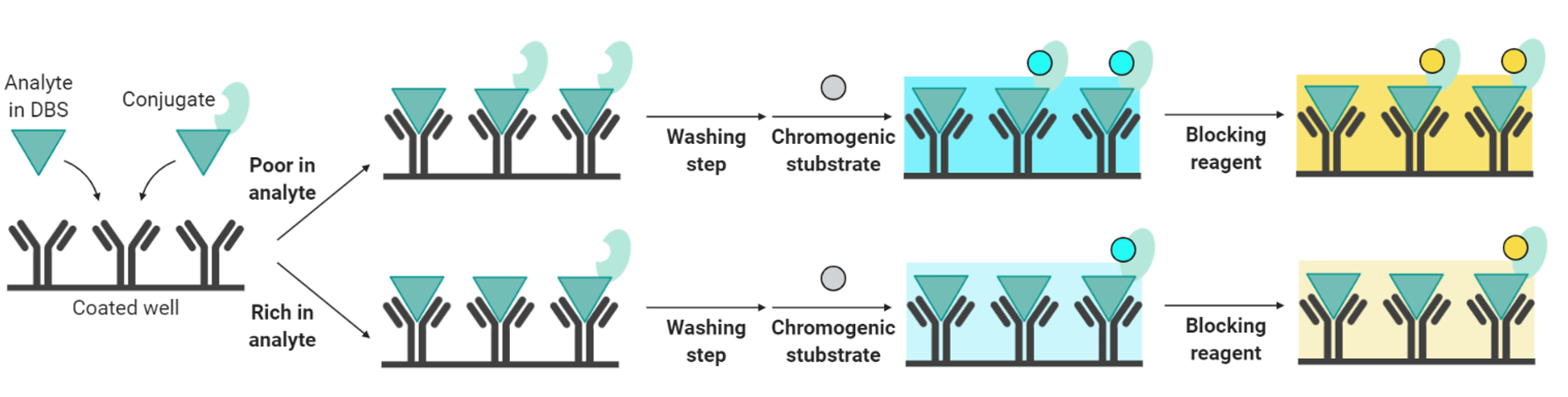
The NEONATAL TSH Screening ELISA kit is an ELISA-type quantitative test for quantifying thyrotropin or thyroid stimulating hormone (TSH) from dried blood lab test samples on 903® or 226 blotting paper. This TSH test is for newborn baby screening for congenital hypothyroidism.