We use cookies to offer you the best experience on our site. You can find out more about the cookies we use or disable them in the Cookie settings
Mass Spectrometry
Targeted MS/MS Hemo
The newborn screening kit for hemoglobinopathy evaluation, Targeted MS/MS Hemo, is a qualitative analytical test for newborn screening for hemoglobin (Hb)-related conditions such as sickle cell disease. This test is based on the detection by mass spectrometry of peptides generated during the digestion of normal Hb and/or variants (HbA, HbA2, HbC, HbDPunjab/Los Angeles, HbE, HbF, HbOArab and HbS) of the patient.
Pathologies Detected
- Alpha-thalassemias test
- Beta-thalassemias test
- Sickle cell disease or sickle cell trait screening – HbS
- Hemoglobin C or trait – HbC
- Asymptomatic or pathological composite heterozygosity for the following Hb variants: HbC, HbDPunjab/Los Angeles, HbE, HbF, HbOArab and HbS.
Test Principle
The principle of the Targeted MS/MS Hemo neonatal hemoglobinopathy evaluation screening test is based on rapid extraction and denaturation of hemoglobin (Hb) from samples, on 903® or 226 blotting paper, of newborns before digestion with trypsin.
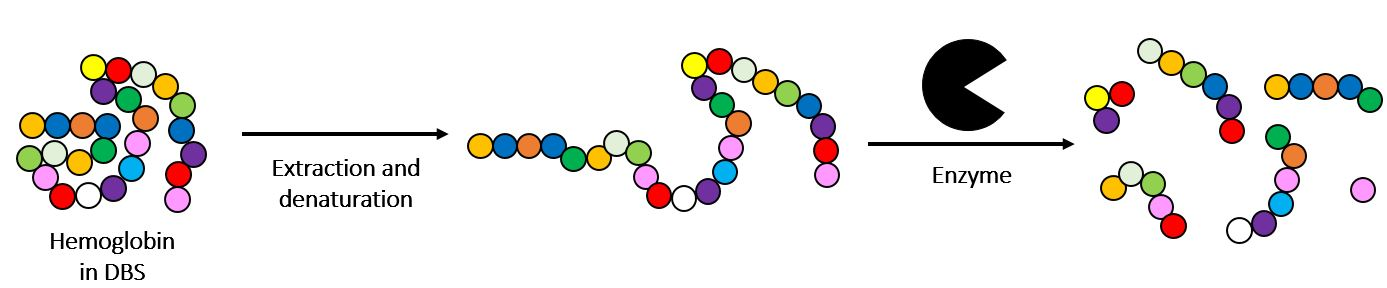
The digested hemoglobin extract is then analyzed by tandem mass spectrometry (MS/MS), by continuous flow injection analysis (FIA), on a “Triple Quadrupole Mass Spectrometer” or equivalent (TQMS). The extract is then ionized by electrospray and the hemoglobin peptides (Hb) are analyzed in “Multiple Reaction Monitoring” (MRM) mode: by peptide, two lead ions produced by collision of the parent ion with an inert gas are selected. 11 peptides are then detected simultaneously by sweeping the two “parent ion/lead ion” pairs specific to each peptide.
After mass spectrometry analysis, the specific ratios are calculated based on the signals generated for the different peptides. They are then analyzed by an algorithm to identify the variants and forms of hemoglobin (Hb) present in the sample. This analysis is carried out via a software to help interpret the results.
The newborn screening kit for hemoglobinopathy evaluation also contains an internal control that meets a dual objective:
- Ensure proper execution of extraction, denaturation and digestion steps;
- Ensure proper operation of the mass spectrometer.
Benefits of the Method
Diseases
This test is intended for neonatal screening of hemoglobinopathies as well as sickle cell disease in newborns, performed using blood samples dried on Dried Blood Spots (DBS), of type 903® or 226.
General Info
Product code :
- J-LS-96 for 96 determinations
- J-LS-480 for 480 determinations