Neonatal MSUD
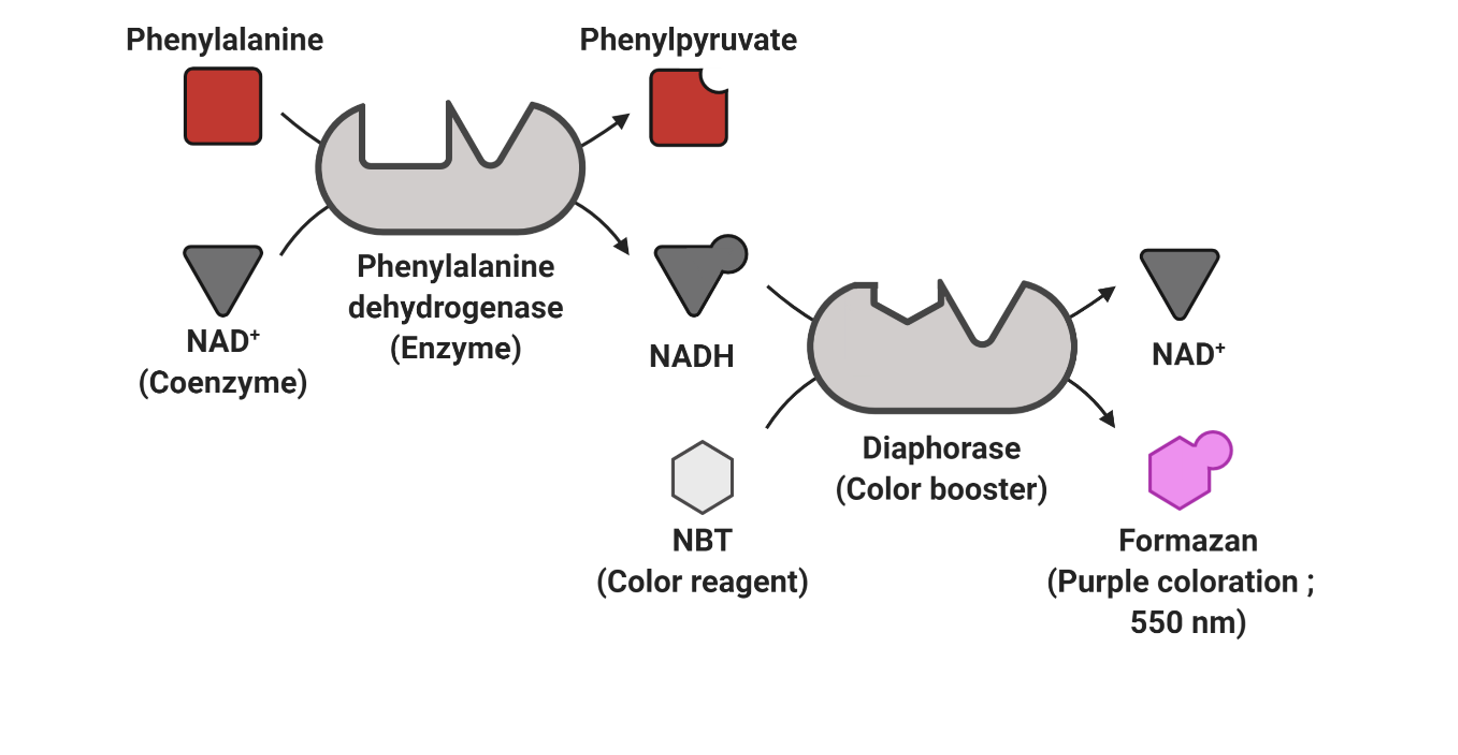
NEONATAL MSUD Screening Assay is a quantitative enzymatic test that assays leukine, one of the 3 amino acids accumulated in maple syrup urine disease (MSUD) also known as ketoacidemia or branched-chain amino acids (BCAAs). This test is for maple syrup urine disease newborn screening, and is performed using dried blood samples on 903® or 226 type blotting paper.