Test Principle
The assay is divided into two steps: a first extraction and lysis step and a second DNA amplification step via qPCR.
The extraction and lysis of the sample on blotting paper is done through the addition of two buffers and a 96-well microplate incubation step.
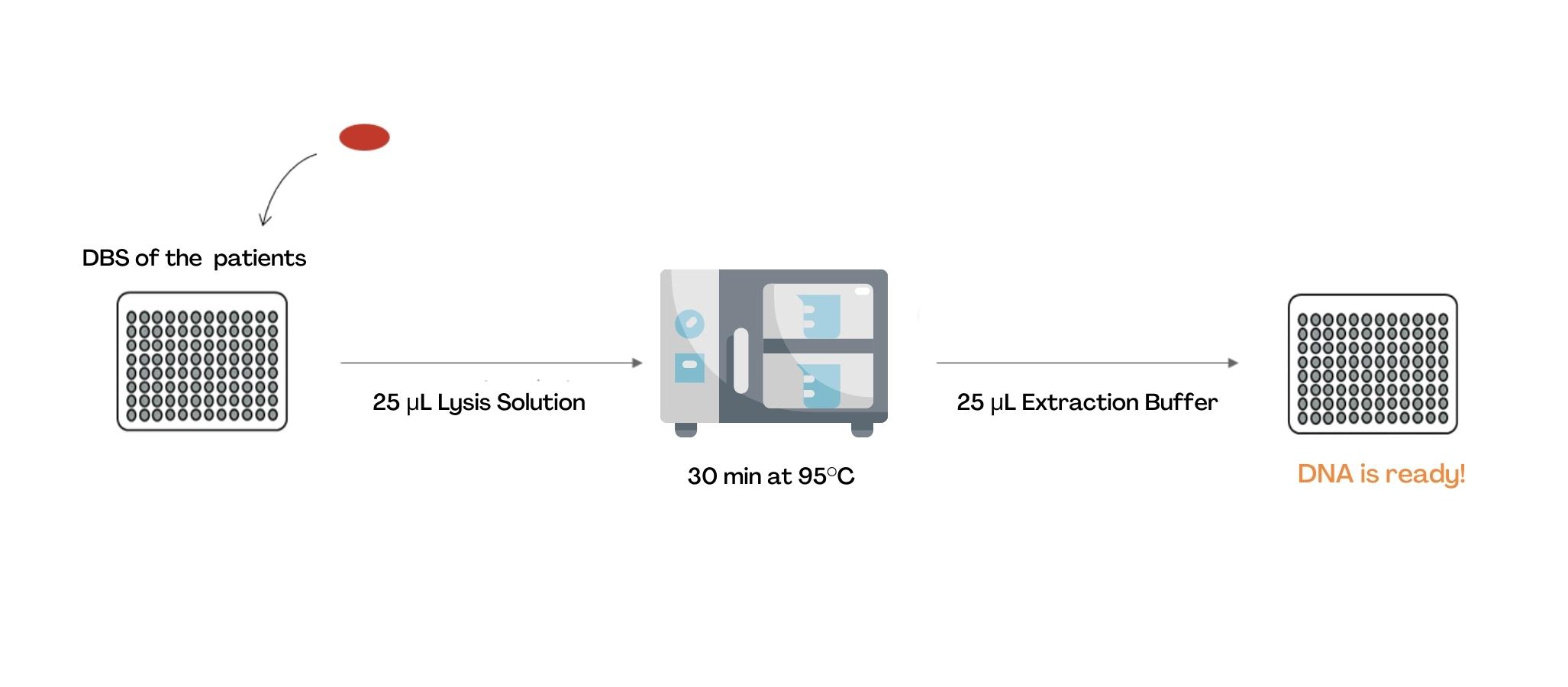
The extracted sample is then transferred into a BioRad microplate, pre-filled with Master Mix, provided in the kit.
The amplification step is performed using the BioRad CFX96 thermocycler and the qPCR program takes approximately 1 hour. The FAM and HEX fluorochromes are used for detection of the non-mutated SMN1 allele and the “home gene” RPP30, respectively.
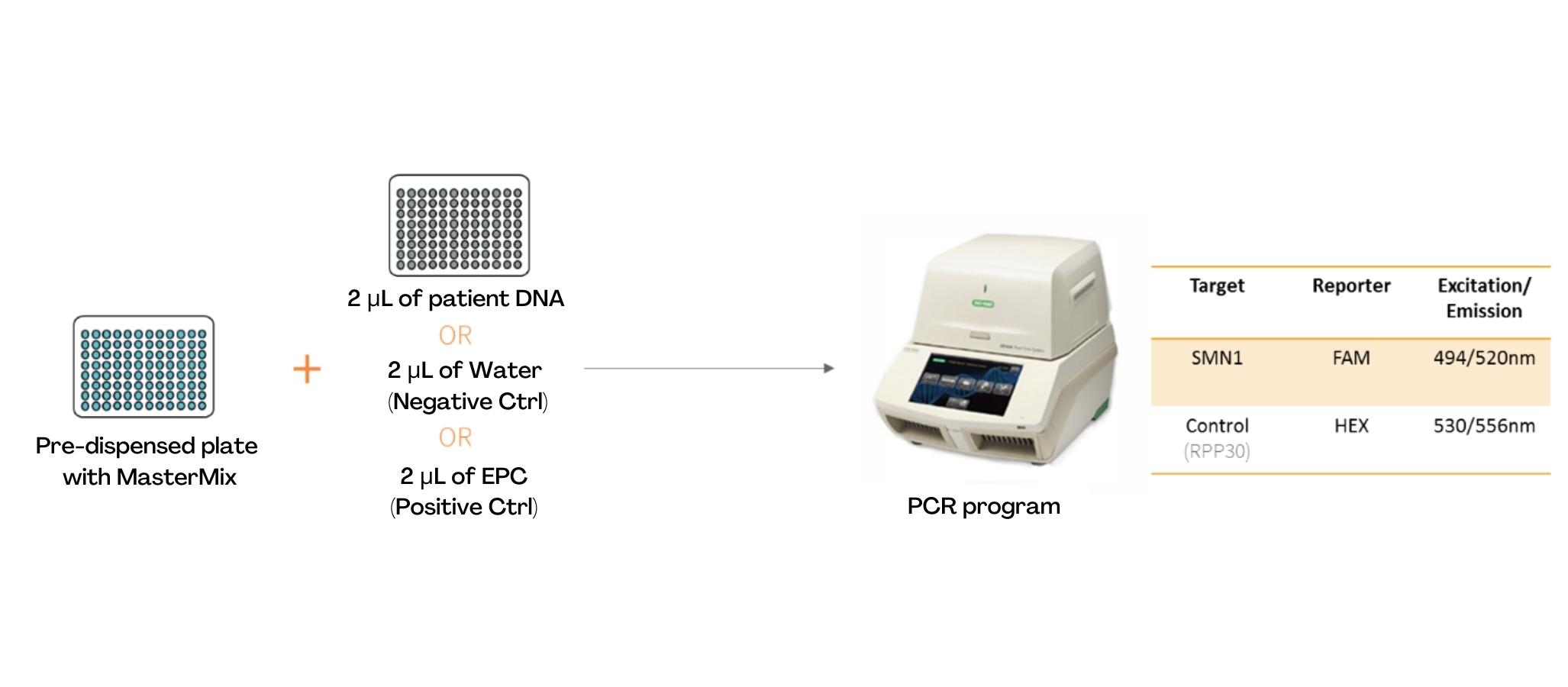
The sample of a healthy or heterozygous patient will produce a HEX and FAM signal and a homozygous sick patient will produce a HEX signal only.
A synthetic DNA control, recognized by HEX and FAM, is provided in the kit to ensure the proper functioning of the amplification step.
Illness
This test is for spinal muscular atrophy (SMA) newborn screening and is performed using dried blood samples on 903® or 226 type blotted paper.
General Info
Product code : K-LT-480 for 480 determinations